Welcome and thank you for your interest and participation in our collaboration with the NH-ISAC, FDA, and MDISS for the MDRAP Risk Assessment of Medical Devices!
Your input is highly valued and greatly appreciated.
The goal of the MDISS - NH-ISAC - FDA collaboration is to improve the security and safety of medical devices by providing tools to assist healthcare delivery organizations (HDOs) reduce their vulnerability footprint in a meaningful way by prioritizing and managing risk assessments to contain resource expenditures. The data collected and shared through the Community "sharing" function will also play a key role in advancing the mission of MDISS and the NH-ISAC by providing threat intelligence data to augment the impact of the vulnerability assessment.
The MDRAP Risk Assessment project is made up of the following four high level, key phases:
- MDRAP Adopter Onboarding
- Pre-Assessment and Assessment Activities
- Post Assessment and Client Support
- Client Engagement and Community Building
Onboarding Process Overview
- MDRAP Demo and Onboarding email.
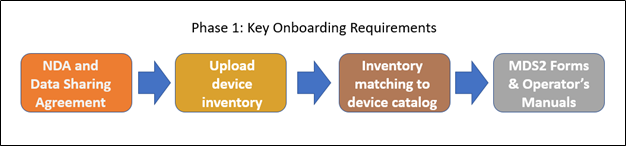
- Complete Non-Disclosure Agreement (NDA) and Collaborative Data Sharing Agreement.
- Collect and share medical device inventory for uploading to MDRAP.
- Inventory upload and matching to the MDRAP device catalog.
- Gather all MDS2s and Operator's Manuals for the inventory of medical devices that will be assessed. Check the MDS2 Library within MDRAP before contacting MDMs to request MDS2 forms - the MDRAP MDS2 Library publicly shares all MDS2 forms that are available.
- Upload all MDS2 forms for connected medical devices that are not already published in the MDS2 Library. This will enable us to continue to grow our Library of MDS2s, which will benefit the entire community of MDRAP users.
The MDISS team of dedicated professionals are here to support you through every step of your medical device risk assessment process. Whether it’s providing relevant online support resources, assessment training, or technical support, our pledge is to support your risk assessment efforts and resolve technical issues in a timely and comprehensive manner.
0 Comments